40%+
Average reduction in length of lyo cycle after R&D support
65%+
Average increase in productivity after R&D support
80%+
Average reduction in cost of storage after R&D support
60%+
Average reduction in cost of production after R&D support
Biopharma Group is ISO 9001 & ISO 13485 Accredited
More Information
R&D Services/Analytical Lab Services
 Biopharma Group’s contract R&D services focus on freeze drying (dried product) and liquid formulation development – allowing us to offer a comprehensive range of expert led CDMO services. Analysis options include comparable process studies, formulation & cycle development, process reviews/audits, optimisation and scale-up that incorporate PAT (Process Analysis Technology) for risk-based quality assurance.
Biopharma Group’s contract R&D services focus on freeze drying (dried product) and liquid formulation development – allowing us to offer a comprehensive range of expert led CDMO services. Analysis options include comparable process studies, formulation & cycle development, process reviews/audits, optimisation and scale-up that incorporate PAT (Process Analysis Technology) for risk-based quality assurance.Our team of CDMO specialists understand the importance of creating an optimised product and process in the early stages of a project development cycle and tailor’s solutions to specific project requirements.
GMP & Non-GMP Production Services
GMP: Available soon, Biopharma Group’s new GMP freeze drying facility is set to provide a much-needed resource to pharmaceutical companies needing small batch clinical production facilities for first-in-human trials. The integration of a new GMP facility into our CDMO services will provide freeze drying for up to 3200 2R vials in our state-of-the-art ATS Hull S10 freeze dryer – alongside full isolation facilities in a Grade A cleanroom environment for the sterile production of both liquid and lyophilized products.
Non-GMP: We offer flexible and scalable CDMO services for clients seeking formulations that don't require the stringent GMP standards. This versatility is valuable for research, development, and production of non-sterile products across diagnostics, pharmaceuticals, and biotechnology. Whether it's pilot-scale development or larger non-sterile production runs, our services adapt to the unique demands of each project.
Lyobeads Processing
 Lyobeads, aka lyophilized spheres, have become a popular technology in the field of freeze drying to the diagnostics industry - as the primary advantage is their flexibility in formulation. They allow for the development of a single formulation that can be easily adjusted for various applications, even in different container types. This versatility can significantly reduce research and development (R&D) investment, making lyobeads an attractive option for companies looking for diagnostic and manufacturing assistance.
Lyobeads, aka lyophilized spheres, have become a popular technology in the field of freeze drying to the diagnostics industry - as the primary advantage is their flexibility in formulation. They allow for the development of a single formulation that can be easily adjusted for various applications, even in different container types. This versatility can significantly reduce research and development (R&D) investment, making lyobeads an attractive option for companies looking for diagnostic and manufacturing assistance.
Biopharma Group has been at the forefront of lyobead processing evolution, supporting many customers to increase productivity whilst achieving significant cost and time savings.
Industry Applications - Diagnostics, Pharma and Biotech
 Diagnostics: Our CDMO specialists are experts in formulation development and lyophilisation processes. This means that the lab teams at Biopharma Group have the capability to support the creation of stable and efficient diagnostic reagents and test kits, enhancing the accuracy and reliability of diagnostic tests.
Diagnostics: Our CDMO specialists are experts in formulation development and lyophilisation processes. This means that the lab teams at Biopharma Group have the capability to support the creation of stable and efficient diagnostic reagents and test kits, enhancing the accuracy and reliability of diagnostic tests.
Pharma: Our QbD methodology and DoE principles are instrumental in optimising drug formulations, enabling pharmaceutical companies to develop products more rapidly and cost-effectively.
Biotech: Our services facilitate the development of biologics, including vaccines and biopharmaceuticals. We contribute to the advancement of biotechnology by providing the necessary expertise and infrastructure for the efficient and compliant production of these critical products.
Quality by Design (QbD) and Design of Experiment (DoE)
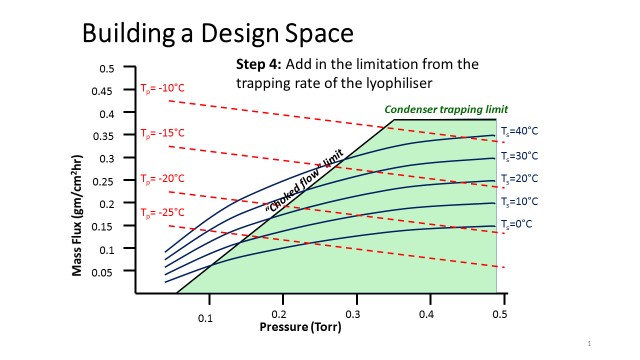 Quality by Design (QbD) is revolutionising pharmaceutical development by replacing traditional trial-and-error methods. As part of our CDMO services Biopharma Group champions this approach - significantly reducing the number of developmental cycles needed for formulation and lyophilisation process optimisation.
Quality by Design (QbD) is revolutionising pharmaceutical development by replacing traditional trial-and-error methods. As part of our CDMO services Biopharma Group champions this approach - significantly reducing the number of developmental cycles needed for formulation and lyophilisation process optimisation.
QbD's precision and predictability benefit a broad spectrum of pharmaceutical products, including small and large molecules and biologics. Biopharma Group's CDMO services aim to define design spaces and critical process parameters, ensuring compliance with stringent regulatory standards.
As CDMO specialists, our expertise results in efficiency and cost savings - exemplified by a 60% reduction in investment for a client's lyophilisation process through utilising a QbD approach.
Low Bio-burden Lab
 Biopharma Group is at the forefront of pharmaceutical research and development, boasting three in-house, state-of-the-art Category D labs. These low bio-burden facilities demonstrate our commitment to precision and safety in lyophilisation processes and the manufacturing of dried and liquid formulations.
Biopharma Group is at the forefront of pharmaceutical research and development, boasting three in-house, state-of-the-art Category D labs. These low bio-burden facilities demonstrate our commitment to precision and safety in lyophilisation processes and the manufacturing of dried and liquid formulations.
Equipped with cutting-edge technology and maintained to the highest industry standards, our Category D labs are the cornerstone of our mission to deliver excellence in pharmaceutical solutions.
ISO 13485 Accreditation
 Our ISO 13485 accreditation is a testament to Biopharma Group's commitment to quality and compliance as a provider of CDMO services. This certification ensures that our processes, from research and development to manufacturing, adhere to the highest global standards.
Our ISO 13485 accreditation is a testament to Biopharma Group's commitment to quality and compliance as a provider of CDMO services. This certification ensures that our processes, from research and development to manufacturing, adhere to the highest global standards.
As a Contract Development and Manufacturing Organisation (CDMO), Biopharma Group's ISO 13485 accreditation is your assurance of quality, precision, and regulatory compliance in every aspect of your project.
With this accreditation, we can seamlessly integrate our services into your lyophilization requirements and samples development, ensuring a smooth and compliant journey from concept to market.
Accreditatons: